Basic Info.
Model NO.
qk-194
Transport Package
PE
Specification
61*38*32.5cm
Trademark
qinkai
Origin
China. Heze
HS Code
9018390000
Production Capacity
300000pieces/Every Day
Product Description
 Basic Info
Basic Info
Model NO.
infusion set
Logo Printing
Without Logo Printing
Medical Device Regulatory Type
Type 1
Trademark
according to customer requirements
Transport Package
Carton
Specification
single packed
Origin
China
Product Description
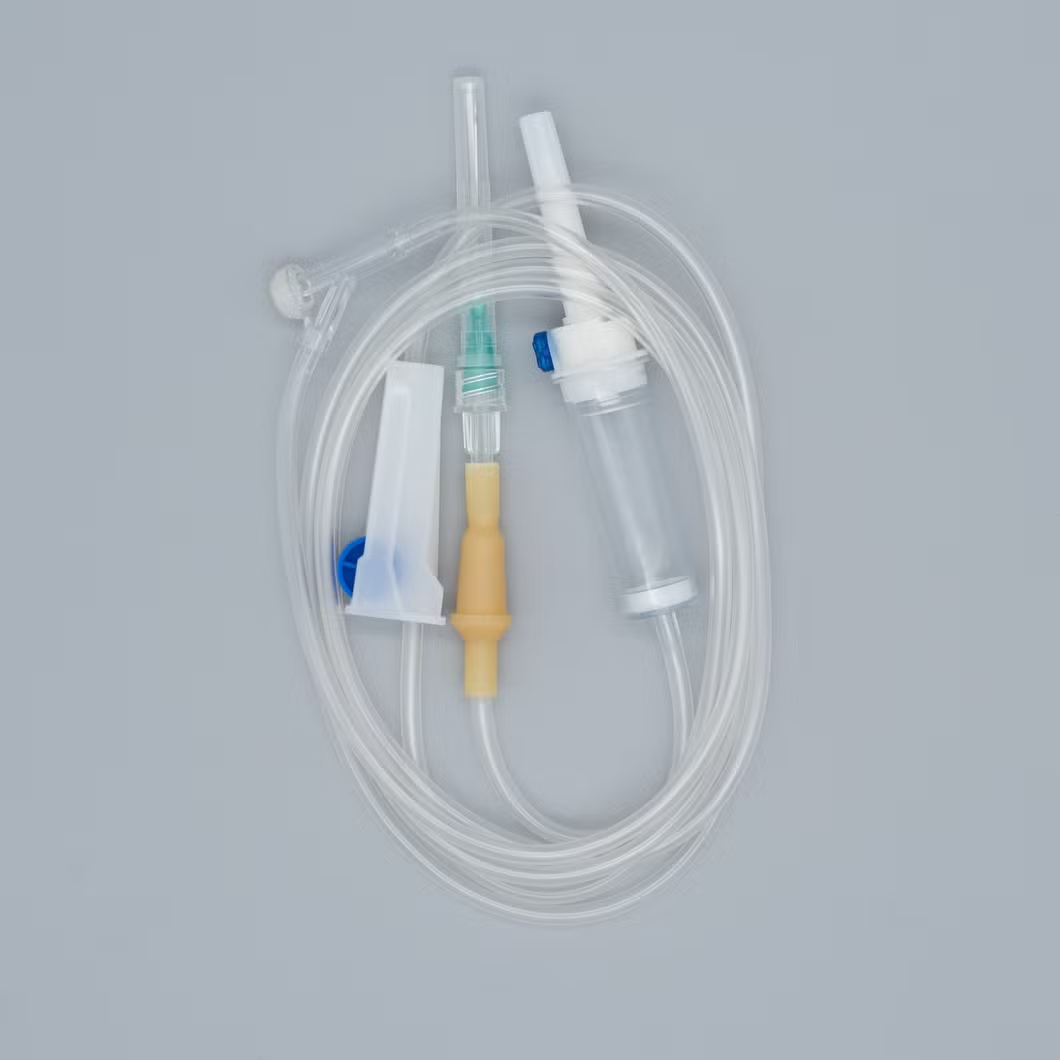
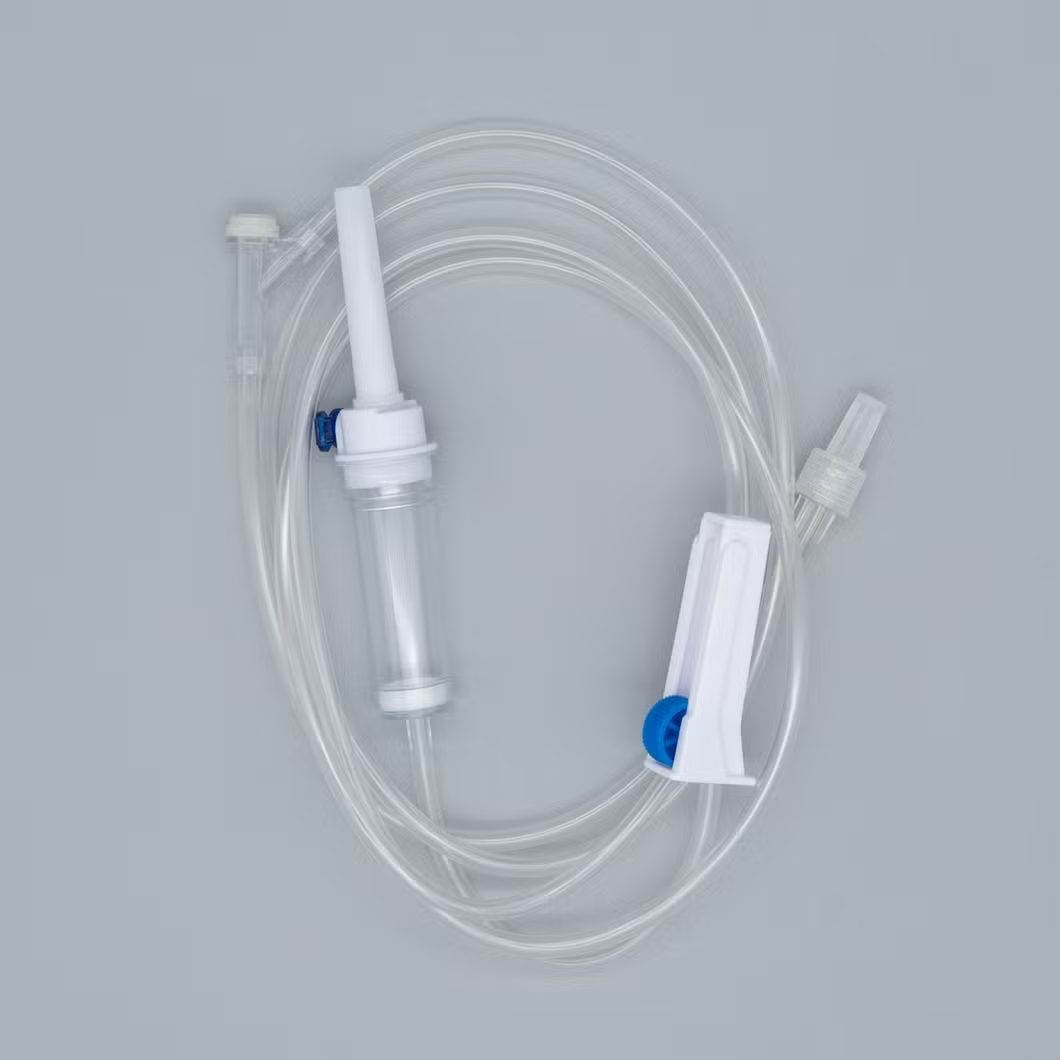
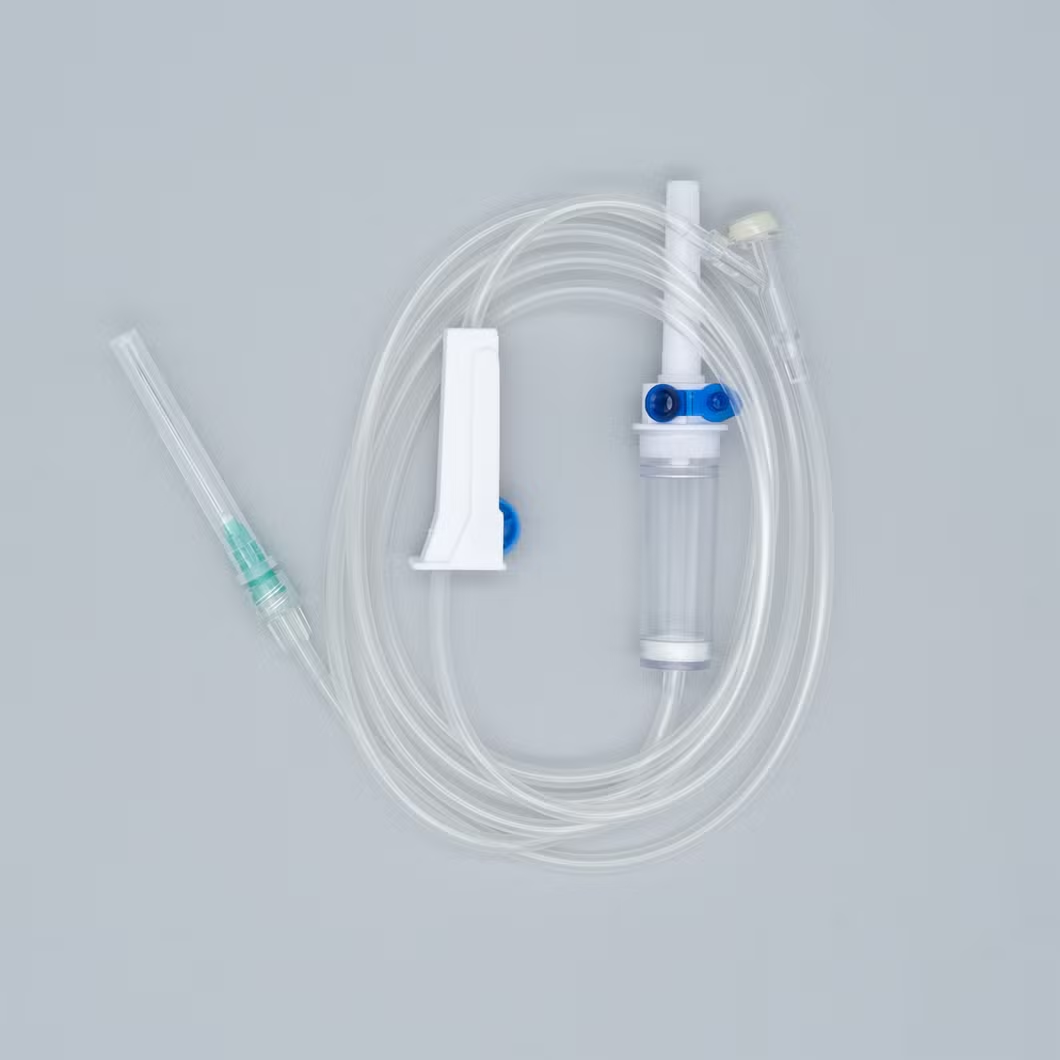
Type: Infusion set Luer lock connector
Consist of:
1. Piercing device--Made of medical grade white ABS, with or without air
Inlet, 3 or 4 holes size according to ISO8536-4 standard.
2. Protective cap--Made of PP or PE.
3. Drip chamber--Made of soft PVC, size according to ISO8536-4
Standards, 4.5cm--7cm in length.
4. Fluid filter--Support is made of ABS or PE, nylon net, mesh size: 15 micron.
5. Air inlet--Cap is made of red, white, or blue PP
6. Water-repellent filter-PP support, hydrophobic filtering paper, made of
Acrylic resin and nylon.
7. Flow regulator--Made of PP or ABS, normal shape, small size or horned Shape, large size.
8. Injection site--Latex cone, rubber cone, synthetic rubber cone, Y site-
Made of hard transparent PVC+injection site made of natural or synthetic Rubber.
9. Soft tubing--Made of soft medical grade PVC, diameter 3X4mm,
150cm and 180cm in length, or adjust according to requirement like as elastic tube.
10. Terminal connector: Made of PE, PVC, luer lock or luer slip.
11. Components assembly: By gluing with solvent cyclohexanone
Specification: They are suited to hard containers and soft bags( without
Air inlet type)
Sterile, non-toxic, pyrogen free, sterilized by EOG.
Certificate: CE, ISO9002, EN46002


Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com



 Basic Info
Basic Info